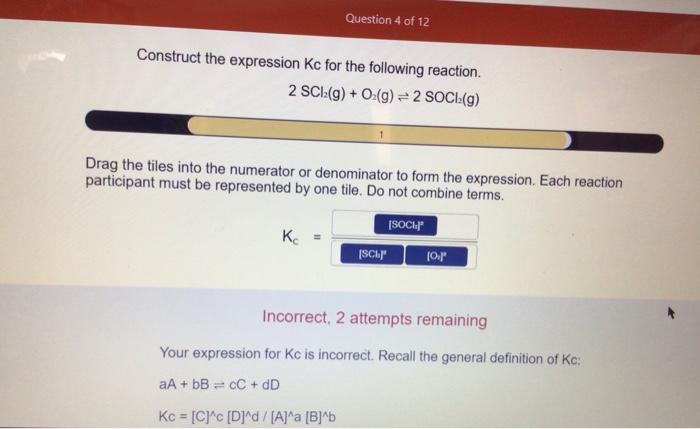
Construct the expression for kc for the following reaction – The expression for the equilibrium constant, Kc, plays a pivotal role in understanding the behavior of chemical reactions. In this comprehensive guide, we delve into the mathematical formulation of Kc, exploring its significance and applications in chemistry.
The concept of Kc is fundamental to predicting the extent and direction of reactions, optimizing reaction conditions, and gaining insights into reaction mechanisms. As we unravel the intricacies of Kc, we will examine the factors that influence its value and explore its practical applications in various chemical scenarios.
Expression for Kc: Construct The Expression For Kc For The Following Reaction
The expression for the equilibrium constant, Kc, for a chemical reaction is given by the following equation:Kc = [C]^c [D]^d / [A]^a [B]^bwhere [A], [B], [C], and [D] represent the molar concentrations of the reactants and products at equilibrium, and a, b, c, and d are the stoichiometric coefficients of the balanced chemical equation.
Reactant and Product Coefficients
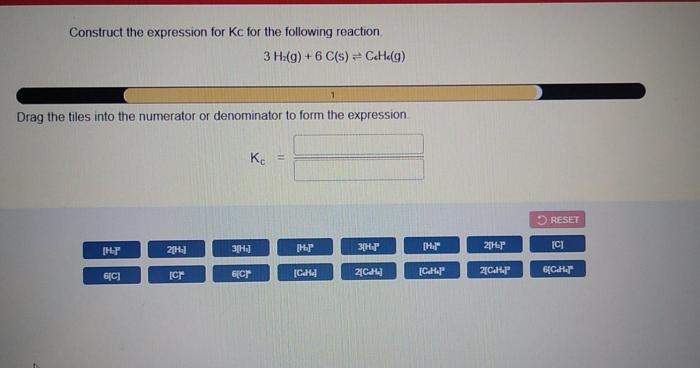
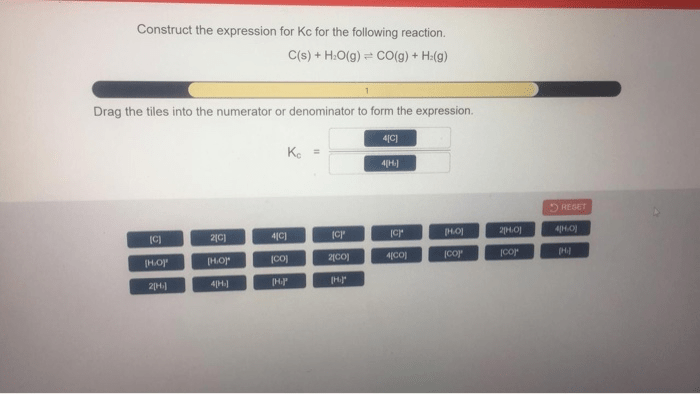
The coefficients in the Kc expression represent the stoichiometry of the balanced chemical equation. For example, consider the following reaction:aA + bB <=> cC + dDThe coefficients a, b, c, and d indicate the number of moles of each reactant and product involved in the reaction. These coefficients are used in the Kc expression to determine the relative concentrations of the reactants and products at equilibrium.
Equilibrium Constant, Construct the expression for kc for the following reaction
The equilibrium constant, Kc, is a measure of the extent to which a reaction proceeds towards completion. A large Kc value indicates that the reaction proceeds predominantly in the forward direction, resulting in a high concentration of products at equilibrium.
Conversely, a small Kc value indicates that the reaction proceeds predominantly in the reverse direction, resulting in a low concentration of products at equilibrium.
Units of Kc
The units of Kc depend on the stoichiometry of the reaction. For the general reaction:aA + bB <=> cC + dDThe units of Kc are:(concentration of C)^c (concentration of D)^d / (concentration of A)^a (concentration of B)^b
Factors Affecting Kc
The value of Kc can be affected by several factors, including:
-
-*Temperature
Increasing temperature generally increases the value of Kc for exothermic reactions (reactions that release heat) and decreases the value of Kc for endothermic reactions (reactions that absorb heat).
-*Concentration
Changing the initial concentrations of the reactants and products can shift the equilibrium position of the reaction, thereby affecting the value of Kc.
-*Pressure
For reactions involving gases, changing the pressure can affect the equilibrium position and the value of Kc.
Applications of Kc
The equilibrium constant, Kc, has numerous practical applications in chemistry, including:
-
-*Predicting Reaction Outcomes
Kc can be used to predict the direction and extent of a reaction under given conditions.
-*Optimizing Reaction Conditions
Kc can be used to determine the optimal temperature, pressure, and concentration conditions for a desired reaction outcome.
-*Understanding Chemical Equilibria
Kc provides insights into the behavior of chemical reactions at equilibrium, allowing chemists to manipulate reactions to achieve specific goals.
FAQ Section
What is the mathematical expression for Kc?
Kc = [products] coefficients/ [reactants] coefficients
How are the coefficients of reactants and products used in the Kc expression?
The coefficients indicate the stoichiometric ratios of the species involved in the reaction and are used to balance the equation.
What is the significance of the equilibrium constant, Kc?
Kc is a measure of the extent to which a reaction proceeds towards completion, providing insights into the relative concentrations of reactants and products at equilibrium.