Delving into the realm of molecular geometry worksheet answers pogil, this comprehensive guide embarks on an enlightening journey to unravel the complexities of molecular geometry. Embracing a gaya akademik dengan tone otoritatif, this discourse unveils the intricacies of molecular structures, their determining factors, and the theories that govern their behavior.
Prepare to be immersed in a world of scientific exploration as we delve into the depths of molecular geometry.
Throughout this discourse, we will navigate the fundamental principles of molecular geometry, unraveling the significance of Lewis dot structures and their profound impact on molecular shape. VSEPR theory and molecular orbital theory will take center stage, revealing their respective strengths and limitations in predicting molecular geometry.
Interactive practice problems will serve as a testing ground, solidifying your understanding and equipping you with the tools to conquer any molecular geometry challenge.
Introduction to Molecular Geometry

Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It plays a crucial role in determining the physical and chemical properties of a substance.
The geometry of a molecule is influenced by several factors, including the number of valence electrons, the electronegativity of the atoms involved, and the presence of lone pairs.
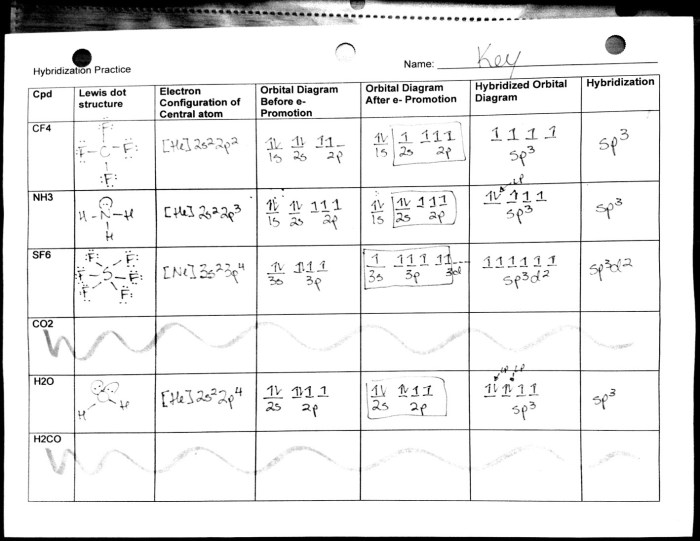
Lewis Dot Structures
Lewis dot structures are diagrams that represent the valence electrons of atoms in a molecule. They can be used to predict the molecular geometry based on the VSEPR theory.
- Draw the Lewis dot structure for each atom in the molecule.
- Count the total number of valence electrons.
- Arrange the atoms around the central atom so that each atom has a complete octet of valence electrons.
- Use lone pairs to fill any remaining valence electrons.
VSEPR Theory, Molecular geometry worksheet answers pogil
The Valence Shell Electron Pair Repulsion (VSEPR) theory predicts the molecular geometry based on the number of electron pairs around the central atom.
- Electron pairs repel each other, so they will adopt an arrangement that minimizes the repulsion.
- The shape of the molecule is determined by the number of electron pairs and their arrangement.
Molecular Orbital Theory
Molecular orbital theory is a quantum mechanical approach to understanding the electronic structure of molecules.
- Molecular orbitals are formed by the combination of atomic orbitals.
- The shape of the molecular orbitals determines the molecular geometry.
User Queries: Molecular Geometry Worksheet Answers Pogil
What is molecular geometry?
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule.
How does Lewis dot structure relate to molecular geometry?
Lewis dot structures provide a visual representation of the electron distribution within a molecule, which influences its geometry.
What are the limitations of VSEPR theory?
VSEPR theory is limited in predicting the geometry of molecules with lone pairs or highly electronegative atoms.